Cancer Immunotherapy
Bladder Cancer
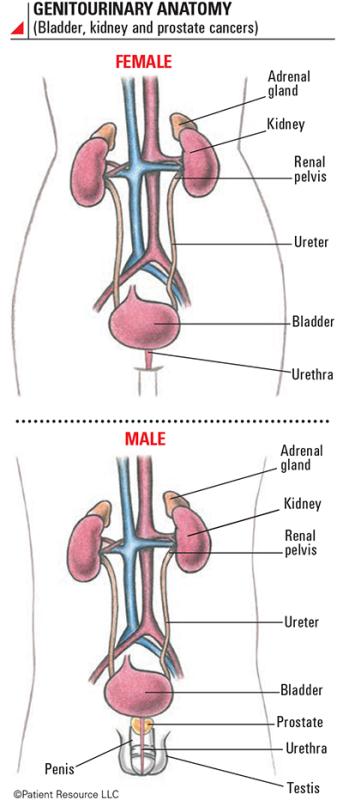
The bladder is a hollow organ in the pelvis, and its primary function is to hold urine before it exits the body. The urine travels to the bladder from the kidneys – where it’s made – through tubes called ureters. The bladder wall is flexible, enabling the bladder to hold approximately two cups (500 cc) of urine. When it’s full, the muscles in the bladder wall contract and force the urine out of the body through a tube called the urethra.
What is Bladder Cancer?
Bladder cancer begins when healthy cells in the bladder lining, most commonly urothelial cells, change and grow uncontrollably, forming a mass called a tumor. A tumor can be cancerous or benign. A cancerous tumor is malignant, meaning it can grow and spread to other parts of the body. A benign tumor means the tumor can grow but will not spread.
The bladder is a hollow organ that stores urine before it exits the body during urination. It can hold approximately two cups (500 cc) of urine. The flexible, muscular bladder wall contracts to move urine out of the body through a tube called the urethra. The bladder wall is composed of four layers: urothelium, lamina propria, muscularis propria and serosa.
The most common type of bladder cancer is urothelial carcinoma, also called transitional cell carcinoma, and it has two subtypes: papillary and flat. Papillary tumors grow from the bladder’s inner lining toward the center of the bladder, and flat tumors grow along the surface of the lining.
Other types of bladder cancer include squamous cell carcinoma, adenocarcinoma and small cell carcinoma. All three are almost always invasive.
Bladder cancer tumors can be classified as one of three types.
- Noninvasive bladder cancer hasn’t penetrated any layers of the bladder.
- Nonmuscle-invasive bladder cancer has grown into the lamina propria layer but not into muscle.
- Muscle-invasive bladder cancer has grown deep into the bladder wall and possibly to tissue outside of the bladder.
Immunotherapy for Bladder Cancer
Immunotherapy is making it increasingly possible for many people with bladder cancers to live longer, better-quality lives because training the immune system to respond to cancer has the potential for a more lasting response that can extend beyond the end of treatment. Multiple types of immunotherapy have been approved for bladder cancers and are detailed in this section.
Your doctor will create your treatment plan based on the location, stage, grade, size and extent of the cancer, whether it is primary or recurrent (has returned), your overall health, personal preferences and other factors. Discussing the goal of treatment and the potential side effects and long-term effects of each therapy will be key to helping you make informed decisions.
Immunotherapy for bladder cancers may be used as first- or second-line therapy. First-line therapy is the first treatment given for a disease. When used by itself, first-line therapy is the one accepted as the best treatment. Second-line therapy is given when the first-line therapy doesn’t work or stops working.
You will need to meet certain criteria to be a candidate for immunotherapy. If you have a pre-existing autoimmune disorder, be sure to discuss it with your doctor. Your doctor may test your blood or tumor for biomarkers that may qualify you for immunotherapy. Likewise, a history of certain infections, such as hepatitis B or C and tuberculosis, are important for your doctor to know before making a decision about your treatment.
Biomarkers are substances, such as genes, proteins or molecules, produced by cancer cells or other cells in the body. Biomarkers are also called tumor markers, molecular markers, biological markers or serum markers. Biomarkers that may be tested in bladder cancers for immunotherapy are PD-L1 expression, and levels of microsatellite instability-high (MSI-H) and mismatch repair deficiency (dMMR).
MSI-H describes cancer cells that have a greater than normal number of genetic markers called microsatellites, which are short, repeated sequences of DNA. Every time a cell reproduces itself, it makes a copy of its genes and DNA. During the process, errors in duplication can be made, much like a misspelled word. The body normally corrects the error, but sometimes it isn’t caught and fixed. It then becomes a mutation that is reproduced in later versions of the cell. Cancer cells that have large numbers of microsatellites may have defects in the ability to correct mistakes that occur when DNA is copied. Research has shown that cancers with MSI-H features respond better to immunotherapy. MSI-H is often tested in certain types of cancer, such as colorectal cancer, but may not always be tested in bladder cancers. You can ask your health care team about testing your tumor for this biomarker.
Immunotherapy is not effective for every person, even if it is approved for that person’s cancer type. Scientists are studying patient responses to immunotherapy to find out why. They are also investigating other methods for using the immune system to fight cancer to improve the effectiveness of this treatment (see Clinical Trials).
The FDA approved the first-ever immunotherapy, bacillus Calmette-Guérin (BCG) for bladder cancer, in 1990. Today, more types of immunotherapy are approved to treat bladder cancer (see Genitourinary Anatomy).
Immune checkpoint inhibitors are approved for urothelial carcinoma, a type of bladder cancer, which is locally advanced (cancer that has spread to nearby tissue or lymph nodes) or metastatic, which has spread to other areas of the body. For some diagnoses, your doctor must perform PD-L1 testing to determine if your level is high enough for an immune checkpoint inhibitor. Some immunotherapy drugs are approved for first-line therapy and others are approved for second-line therapy.
You may qualify for first-line therapy if your tumors express high levels of PD-L1 as determined by an FDA-approved test or if you cannot receive chemotherapy. If you meet any of the following criteria, you may qualify for an immune checkpoint inhibitor as a second-line therapy for urothelial carcinoma:
- You cannot receive any platinum-containing or cisplatin chemotherapy regardless of PD-L1 status.
- You previously had chemotherapy but the cancer returned.
- You have disease progression after prior treatment, have no other treatment alternatives and your tumor tests positive for microsatellite instability-high, deficient mismatch repair or tumor mutational burden-high.
Also approved is a conjugated monoclonal antibody (mAb), meaning it combines two types of drugs: an antibody and a chemotherapy. The antibody allows the drug to target specific receptors on a cancer cell and then delivers the chemotherapy directly to it. This mAb is approved for locally advanced or metastatic urothelial cancer in patients who previously received an immune checkpoint inhibitor and a platinum-containing chemotherapy. It is considered a second-line therapy.
Cytokines and modified bacteria, two types of nonspecific immune stimulation, are also approved to treat urothelial cancer. The two types of cytokines are interleukins and interferons, which are some of the first types of immunotherapy approved.
A modified bacteria has been changed to ensure it will not cause an infection while stimulating an immune response. This modified bacteria for bladder cancer is the first immunotherapy ever approved and continues to be one of the main treatments for nonmuscle-invasive bladder cancer.
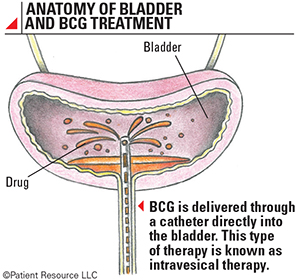
The modified bacteria is a weakened version similar to the bacterium that causes tuberculosis. It is delivered directly into the bladder through a catheter. This is called intravesical therapy (see Anatomy of Bladder and BCG Treatment). The drug attaches to the inside lining of the bladder and stimulates the immune system to destroy the tumor. It is approved for early-stage bladder cancer and to reduce the risk of recurrence in noninvasive bladder cancers.
Types of Approved Immunotherapies
The U.S. Food and Drug Administration (FDA) approved the first-ever immunotherapy in 1990, and it was bacillus Calmette-Guérin (BCG) for bladder cancer. It was considered a breakthrough for modern immunotherapy and is now a standard-of-care treatment. Today, more types of immunotherapy are approved to treat bladder cancer.
Immune Checkpoint Inhibitors
This class of immunotherapy blocks PD-1 and PD-L1 from connecting, which prevents the immune system from slowing down its attack on cancer. These medications are given intravenously (IV) through a needle inserted into a vein in your arm and are considered systemic, meaning they travel throughout your body.
Immune checkpoint inhibitors are approved for urothelial carcinoma, a type of bladder cancer. These are approved for bladder cancer that is locally advanced (cancer that has spread to nearby tissue or lymph nodes) or metastatic, which has spread to other areas of the body. For some diagnoses, your doctor must perform PD-L1 testing to determine if your level is high enough for an immune checkpoint inhibitor. Some are approved for first-line therapy and others are approved for second-line therapy.
You may qualify for first-line treatment if your tumors express PD-L1 or you cannot receive chemotherapy. If you meet any of the following criteria, you may qualify for an immune checkpoint inhibitor as a second-line therapy:
- You cannot receive any platinum-containing or cisplatin chemotherapy regardless of PD-L1 status.
- You previously had chemotherapy but the cancer returned.
- You have had disease progression following prior treatment, have no other treatment alternatives and test positive for microsatellite instability-high (MSI-H) or mismatch repair deficiency (dMMR).
Monoclonal Antibodies
This type of immunotherapy was recently approved for bladder cancer. The monoclonal antibody (mAb) approved for urothelial carcinoma is a conjugated mAb, meaning it combines two types of drugs: an antibody and a chemotherapy.
The antibody allows the drug to target specific receptors on a cancer cell and then delivers the chemotherapy directly to it. This mAb is approved for locally advanced or metastatic urothelial cancer in patients who previously received an immune checkpoint inhibitor and a platinum-containing chemotherapy. It is considered a second-line therapy.
Non-specific Immune Stimulation
Two classes of this type of immunotherapy have been approved by the FDA for treating urothelial cancer: cytokines and modified bacteria (see Treatment Options).
Two types of cytokines are approved for use in urothelial cancer: interleukins and interferons, which were some of the first types of immunotherapy approved.
The other immunotherapy is a modified bacteria that has been changed to ensure it will not cause an infection while stimulating an immune response. This modified bacteria for bladder cancer was the first immunotherapy ever approved. It continues to be one of the main treatments for nonmuscle-invasive bladder cancer.
The modified bacteria is a weakened version similar to the bacterium that causes tuberculosis. It is delivered directly into the bladder through a catheter. This is called intravesical therapy. The drug attaches to the inside lining of the bladder and stimulates the immune system to destroy the tumor. It is approved for early-stage bladder cancer and to reduce the risk of recurrence in noninvasive bladder cancers, commonly after surgery to remove the tumors.
Clinical Trials
Research to find other forms of immunotherapy for bladder cancer is currently underway, and new therapies may be approved in the future. One focus is on drugs that block CTLA-4, an immune checkpoint pathway (see Treatment Options).
Investigators continue to evaluate multiple types of immunotherapy for treating earlier stages of bladder cancer and recurrent cancer. Ask your doctor if you should consider a clinical trial (see Clinical Trials).



