Cancer Immunotherapy
Lung Cancer
Learning you have lung cancer is life-changing. Give yourself time to digest the news, and then focus on learning as much as you can about your exact diagnosis. Knowing key information, including stage and any biomarkers, will better prepare you to make well-informed decisions with your doctor. If you’re unsure of your specific diagnosis, have your doctor or nurse navigator write it down for you, and be sure to ask about any medical terms you don’t understand.
Explaining Lung Cancer
Your lungs are a pair of large, spongy, expandable organs in your chest cavity that are surrounded by a thin layer of protective tissue (pleura). The right lung is a little larger with three parts (lobes), and the left lung has only two (see lung anatomy illustration below). When you inhale, your lungs absorb oxygen, which is then delivered to the rest of your body. When you exhale, your lungs rid the body of carbon dioxide. Your diaphragm helps your lungs expand and contract when you breathe.
Lung cancer often begins in the lining of the airways when abnormal cells grow out of control, dividing faster and living longer than normal cells. Over time, these cancerous cells accumulate to form a tissue mass (primary tumor). Left untreated, a primary tumor may grow into the pleura and form secondary tumors nearby. The cancer cells can eventually crowd out the normal cells to make breathing increasingly difficult.
.jpg)
In advanced disease, lung cancer cells may break away to form tumors in the opposite lung and distant sites such as the liver, brain or bones. These are known as metastases. Regardless of location, the metastases are still considered lung cancer and are treated as such.
Your doctor will analyze the cancer cells in a biopsy specimen or fluid taken from the lung or elsewhere to define the type of lung cancer, which will help guide treatment decisions.
Adenocarcinoma is the most common subtype diagnosed, especially in never smokers. It generally begins in the mucus-producing cells in the more distal (those that are farthest away) airways. Because it tends to grow more slowly than other types of lung cancer, adenocarcinoma is slightly more likely to be found before it spreads. Adenocarcinomas tend to develop in the peripheral lung and spread to distant sites more often than other types except for small cell lung cancer.
Squamous cell lung cancer (epidermoid carcinoma) is the second most common and starts in the early versions of squamous cells, the thin, flat cells that line the more central airways in the lungs. It most often develops in smokers and in the central lung. It spreads to distant sites less often than adenocarcinomas.
Large cell lung cancer can develop anywhere in the lungs and tends to grow and spread quickly. When it includes neuroendocrine features, it may behave and be treated like small cell lung cancer.
Adenocarcinoma and squamous cell and large cell lung cancers are sometimes collectively referred to as non-small cell lung cancer (NSCLC), which accounts for the majority of lung cancer diagnoses. Each type has distinct characteristics and responses to treatment, which makes it important for your doctor to determine the specific type.
Small cell lung cancer (SCLC) is an aggressive form of lung cancer that is defined as limited-stage (confined to one part of the chest, in just one part of the lung and in nearby lymph nodes) or extensive-stage (spread to other parts of the body, such as the bone, brain or other lung).
Immunotherapy for Lung Cancer
The ability to harness the potential of the immune system to fight cancer is giving new hope to people with lung cancer, improving patient outcomes and offering an enhanced quality of life. Sometimes referred to as biologic therapy or biotherapy, immunotherapy trains the immune system to respond to and attack cancer. This type of treatment is very different from conventional options, such as surgery, chemotherapy and radiation therapy, because it has the potential for a more lasting response that can extend beyond the end of treatment.
Treating lung cancer with immunotherapy is considered a significant leap forward, especially because many cases are diagnosed at an advanced stage. The first immunotherapy approval for a type of lung cancer occurred in 2015. Now, immunotherapy options are available for advanced and metastatic non-small cell lung cancer (NSCLC) and extensive-stage small cell lung cancer (SCLC).
Used alone or in combination with other treatments such as chemotherapy, targeted therapy and radiation therapy, immunotherapy may be used as first- or second-line therapy. A first-line therapy is the first treatment given for a disease. When used by itself, first-line therapy is the one accepted as the best treatment. Second-line therapy is given when the first-line therapy doesn’t work or stops working.
Introducing Immune Checkpoint Inhibitors
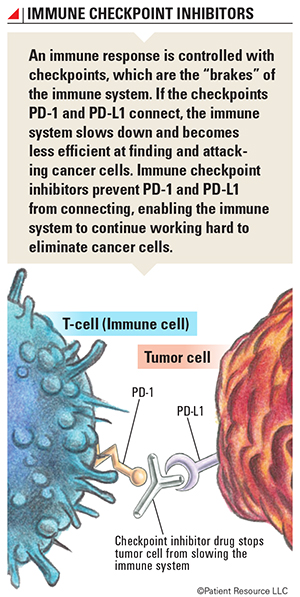
Immune checkpoint inhibitors are the type of immunotherapy currently approved to treat lung cancer. They target the proteins PD-1 (programmed cell death protein 1) and PD-L1 (programmed cell death-ligand 1) found on cells and boost the immune system’s cancer-fighting response. Other types of immunotherapy approved for other cancers are being studied as possible lung cancer treatments (see Clinical Trials).
A primary function of the immune system is to determine which cells or substances are self or non-self. The immune system only makes enough white blood cells to fight non-self cells, also called antigens, present in the body. When the immune system is alerted to the threat of antigens, such as bacteria or viruses, it ramps up production of T-cells that attack and destroy the antigens. After an attack, the immune system must slow down so that the T-cells don’t begin attacking healthy cells. It does this through the use of checkpoints.
Checkpoints keep the immune system “in check.” This process happens between proteins and receptors at the cellular level. To understand how this occurs, it’s important to know that the surface of each cell is not completely round and smooth. Cells are covered with receptors and proteins, which work like puzzle pieces. Proteins have “tabs” that stick out, and receptors have “spaces” that curve inward. When the puzzle pieces fit together (known as binding), chemical signals and information are exchanged in a biochemical reaction; this process allows cells to communicate with each other. When the correct proteins and receptors connect, a series of signals is sent to the immune system to slow down once an immune response is finished.
The immune system also can attack and kill cancer cells. In order for a cancer to grow, it must turn off the immune response. This occurs when cancer cells express immune checkpoint proteins. Three checkpoint receptors that slow down the immune system have been identified, and two are present in lung cancer. When they combine, the reaction signals it’s time to slow down.
- PD-1 is a receptor involved with telling T-cells to die and to reduce the death of regulatory T-cells, which slow down the immune system after an immune response and inhibit T-cells that attack normal, healthy cells that weren’t eliminated before leaving the thymus. PD-1 can tell the immune system to slow down only if it connects with PD-L1.
- PD-L1 is a protein that, when combined with PD-1, sends a signal to reduce the production of T-cells and enable more T-cells to die.
The goal of immune checkpoint inhibitors is to prevent PD-1 and PD-L1 from connecting so that the immune system does not slow down. Checkpoint inhibitor drugs prevent these connections by targeting and blocking PD-1 or PD-L1, and the immune cells continue fighting the cancer. If either checkpoint is blocked, the immune system will not slow down and T-cells can continue to attack antigens and cancer cells.
However, cancer is smart and tries to hide from the immune system. One of the ways a cancer cell can outsmart the immune system is by producing PD-L1 on its own surface and using it as camouflage so that T-cells will see it as a normal cell. T-cells expect only normal cells to produce PD-L1, so when a T-cell encounters PD-L1 on a cancer cell, it is tricked into signaling the immune system to slow down. When an immune checkpoint inhibitor is given, it’s as if the immune system develops X-ray vision and sees through the cancer cell’s camouflage. This keeps the immune response from slowing down and also helps the immune system recognize cancer cells as foreign cells.
Although cancer cells can be clever, the immune system has a long memory when it comes to battling dangerous cells. When your immune system encounters a virus, such as chickenpox, the memory T-cells check to see if that virus has any characteristics of cells they have attacked in the past. If so, your memory T-cells offer you immunity from that virus, and most of the time you don’t get chickenpox again. The memory T-cells alert the rest of the immune system, telling it to make more immune cells to attack the virus and keep you from getting the disease again. Memory T-cells stay alive and store this information for a long time, remaining effective long after treatment ends. Investigators believe that effective immunotherapy can result in cancer-specific memory cells that provide long-term protection against cancer.
Molecular Testing: Biomarkers in Immunotherapy for Lung Cancer
Cancer is caused by mutations in your genes, which are pieces of DNA. Research has found several specific genetic mutations that lead to lung cancer. Doctors can test for these mutations as part of the diagnostic process by looking for biomarkers, which are the molecules produced by the cancer cells or other cells in the body in response to cancer. Testing for biomarkers is known as molecular testing. It may include testing for specific genes, proteins or molecules of the tumor and can be measured in the blood, plasma, urine, cerebrospinal fluid or other body fluids or tissues. Biomarkers are also known as tumor markers, molecular markers, biological markers or serum markers.
Most molecular testing for lung cancer looks for epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) fusions, for which several targeted therapies have been developed. Researchers have identified other biomarkers that are involved in some types of lung cancers. National guidelines currently recommend the following genes be tested if lung cancer is suspected: ALK, BRAF, EGFR, MET, ERBB2 (HER2), NTRK, RET and ROS1.
Next-generation sequencing (NGS) is used to test these genes. This technique is capable of processing multiple DNA sequences simultaneously with more speed and accuracy. NGS can be done on both tumor tissue and blood and, at present, can detect abnormalities associated with specific therapies.
To find out if you are a candidate for immunotherapy, your doctor may also look for the following factors along with molecular testing:
- PD-L1 expression may be tested to determine if the tumor cells or immune cells in the tumor’s microenvironment contain a higher level, which may mean you could be a good candidate for immune checkpoint inhibitors.
- Tumor mutational burden (TMB) is an assessment of the number of genetic mutations in a tumor. It can also help doctors determine if you will respond to immunotherapy. It is believed that the higher the TMB level is, the more likely you will be to respond.
- Microsatellite instability-high (MSI-H) or deficient mismatch repair (dMMR) may be tested to determine if the cancer is caused by genes that have problems repairing themselves. MSI-H describes cancer cells that have a greater than normal number of genetic markers called microsatellites, which are short, repeated sequences of DNA. Every time a cell reproduces itself, it makes a copy of its genes and DNA. During the process, errors in duplication can be made, much like a misspelled word. The body normally corrects the error, but sometimes it isn’t caught and fixed. It then becomes a mutation that is reproduced in later versions of the cell. Cancer cells that have large numbers of microsatellites may have defects in the ability to correct mistakes that occur when DNA is copied. Cancers with MSI-H features appear to respond better to immunotherapy.
Research has shown that people with a high level of PD-L1 expression also typically respond better to immunotherapy. In addition, the level of expression will help your doctor determine whether to give immunotherapy alone or in combination with chemotherapy. People who have tumors with 50 percent or more PD-L1 expression are usually considered good candidates for immunotherapy as a single treatment. If the amount is less than 50 percent, people may be treated with a combination of one or two types of chemotherapy along with an immunotherapy. However, not everyone with a high PD-L1 expression should be given immunotherapy, especially someone with an active EGFR mutation.
Not all people who receive immunotherapy respond. In some cases, people with high expressions of PD-L1 do not respond to immunotherapy. Some people with a low PD-L1 expression do respond but less often.
Researchers are not sure why this happens and more research is needed so immunotherapy is not given to someone who may not respond to it.
Inform your doctor if you have an autoimmune disorder, such as Crohn’s disease, ulcerative colitis or lupus. An autoimmune condition means you have an overactive immune system, and introducing immunotherapy may increase potential safety risks and life-threatening toxicities. Also let your doctor know if you've received immunotherapy before because previous treatments may affect your doctor’s treatment decisions.



